
The RIVER trial is evaluating a potential new therapy, oral nalbuphine ER (extended-release), in reducing chronic cough.
There is no cost to participate, and you will receive medical care from a dedicated study team which consists of doctors and nursing staff.
What will happen in the RIVER trial?
In this trial, all participants will have the opportunity to receive oral nalbuphine ER during a portion of the trial. Some participants will receive placebo first before receiving oral nalbuphine ER. Other participants will receive oral nalbuphine ER first before taking placebo.
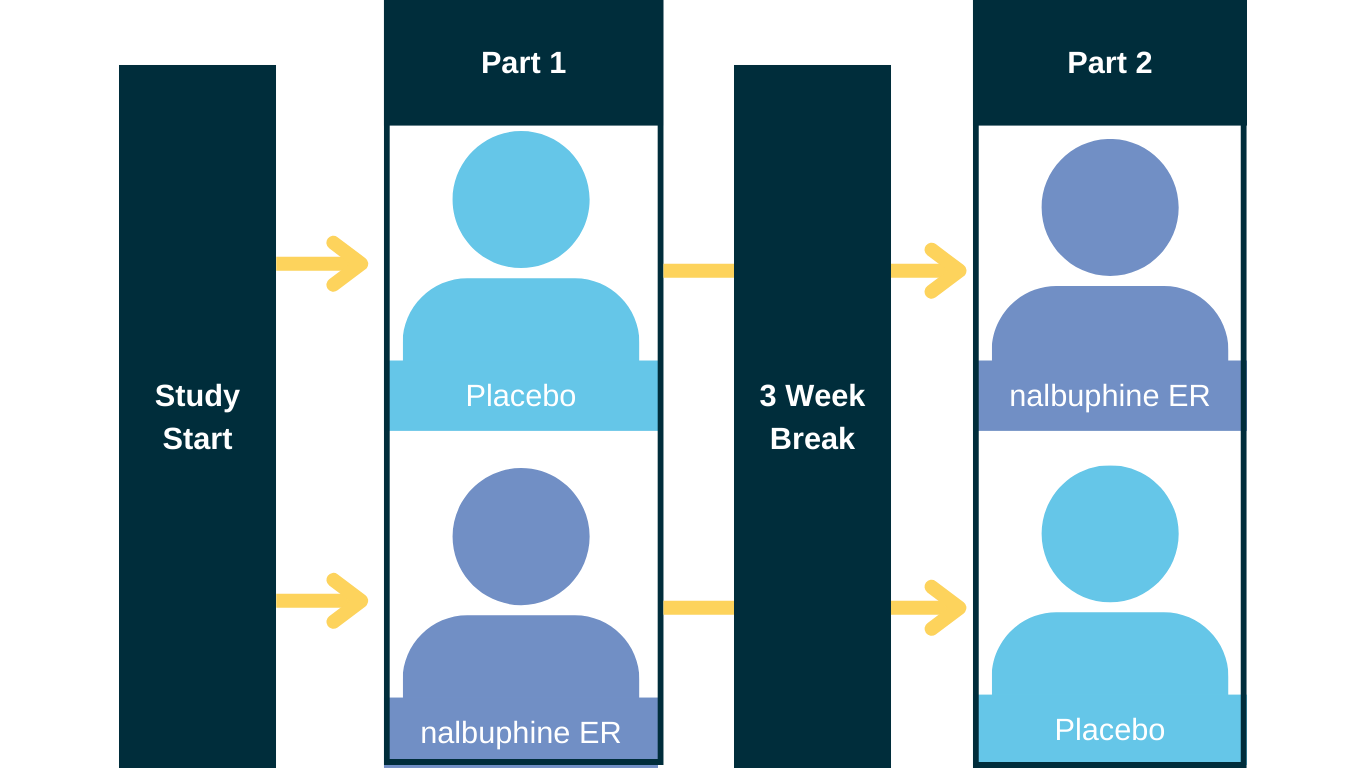
How long is the RIVER trial?
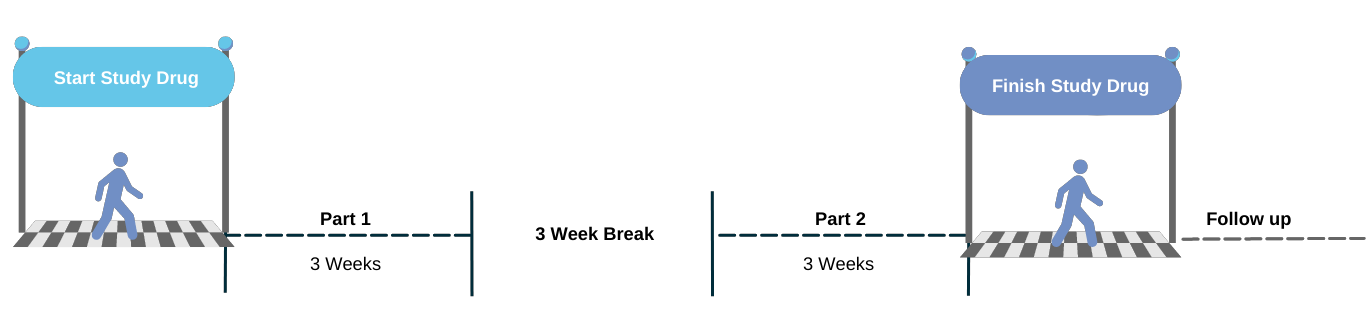
The study includes screening, treatment, washout, and follow up. Participants will take study drug for 6 weeks. After you complete screening, the first part of the study will include taking nalbuphine ER or placebo for 3 weeks. You will then take a 3-week break in which you will not take any study drug. After this break, you will enter the second part of the study where you will take nalbuphine ER or placebo for 3 weeks.
During each part where you are taking a study drug, you will have 4 study visits and 1 telephone call. The study will pay for transportation (this can include personal car mileage, taxi, train, or rideshare) and parking costs for visits to the site. In-person visits ensure that the safety and well-being of participants is being protected.
Why should I join the RIVER trial?
As with all trials with new therapies, there is no guarantee that treatment will work for you. However, oral nalbuphine ER was shown to reduce chronic cough by 75% in a different population. Continued research of nalbuphine ER may help you and other RCC patients in the future.

Study Sites Near You
If you are not located near a study site but are willing to travel, study site staff will discuss travel assistance.

Frequently Asked Questions
What is a clinical trial?
A clinical trial is medical research to learn more about how a potential new medication or how an existing medication works. The research helps to answer important questions on its efficacy and its safety.
All medications must be tested in research studies before participants can receive the medication from their doctor. Strict rules and regulations ensure that clinical research studies are closely monitored and that all study participants are treated ethically and fairly.
Who conducts a clinical trial?
Clinical trials are run by a research team that may include doctors, nurses, and other healthcare professionals.
This clinical trial, like most, is paid for by a pharmaceutical company.
Why is it important to participate in clinical trials?
Clinical trials can only take place when patients with a certain disease or condition choose to participate. Examples of reasons patients choose to join clinical trials include:
1. To play a more active role in their own health care
2. To gain deeper understanding about their disease
3. To potentially gain access to new medical treatments before they are available to the wider public
4. To help find new or better treatments
What is refractory chronic cough (RCC)?
Refractory chronic cough is a consistent cough that may be due to asthma (non-asthmatic eosinophilic bronchitis), gastroesophageal reflux disease (GERD), and postnasal drip syndrome (upper airway cough syndrome) that does not respond to ordinary methods of treatment OR where no cough associated conditions can be identified. ~10% of adults around the world suffer from chronic cough and patients see a number of specialties to try and treat this condition.
Individuals have different ranges of how much they cough per day and how severe their cough is.
How will I take the medication?
The medication will be taken twice daily as follows:
– 2 small tablets taken orally in the morning
– 2 small tablets taken orally in the evening
What is nalbuphine ER and how does it work?
Nalbuphine ER is an oral medication being studied for the treatment of chronic cough. Nalbuphine ER may help decrease the number of times a person coughs by working in the lungs and central nervous system.
What side effects are common with nalbuphine ER?
Nalbuphine ER has been tested in over 1,000 people in different conditions. The most common side effects associated with nalbuphine ER have been vomiting, constipation, dizziness, headache, and sleepiness, which mainly occurred in the first 2 weeks, tended to be mild to moderate, and usually went away in 3-5 days.
Are there any potential risks if I join the RIVER trial?
Your safety is a priority. Your study team will explain all the potential benefits and risks involved with participation in the trial, and you will have the chance to consider this carefully before deciding whether or not to participate.
Thank you for considering participation in the RIVER clinical trial for refractory chronic cough (RCC).
Click on the button below to access a reminder card when you speak to your doctor about the RIVER trial.
